What are the goals of this study?
- To learn about the effects of the COVID-19 vaccines in people with MS, including how they work and what the side effects are
- To produce information that can help guide people with MS and their healthcare providers in making decisions related to COVID-19 vaccination
Who is leading the study and what was the funding source?
The study is being conducted by the iConquerMS research team in collaboration with Dr. Farren Briggs (Case Western Reserve University) and Dr. Farrah Mateen (Massachusetts General Hospital). A steering committee that includes people with MS is guiding and overseeing the study. The project is being funded by the National MS Society and Quest Diagnostics.
How is the study being conducted?
All iConquerMS members are eligible and welcome to participate. Currently there are over 1,500 participants. We ask participants to answer a few surveys on the iConquerMS portal at different timepoints asking about:
- Details of the vaccine that was received (e.g., date received and vaccine manufacturer)
- Short-term reactions to the vaccine (e.g., arm soreness)
- MS symptoms (type of symptom and severity) before and after receiving the vaccine
- COVID-19 infection (for anyone who becomes infected with COVID-19 after vaccination)
- Demographics (e.g., date of birth, race, ethnicity)
- MS characteristics (e.g., type of MS, MS therapies)
We are also inviting some COVER-MS participants to provide blood samples for a substudy. This substudy will analyze the immune responses (antibodies and T-cell activity) to the vaccines to learn how these responses are affected by MS drugs and other factors.
What are we learning from this study?
Our initial study findings have to do with reactions (side effects) to the vaccines. We have learned that: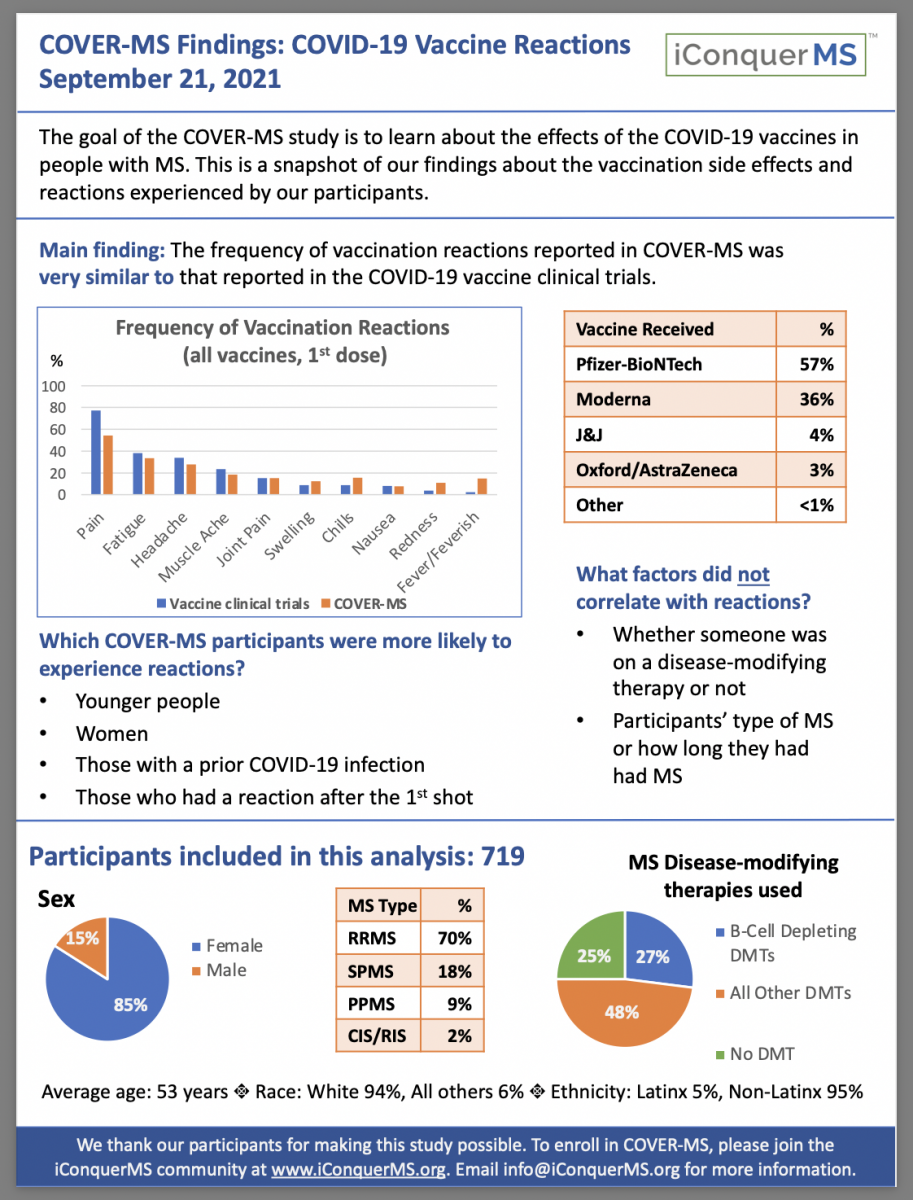
-
The frequency of vaccination reactions reported in COVER-MS was very similar to that reported in the COVID-19 vaccine clinical trials
-
Pain was the most commonly reported reaction, followed by fatigue and headache
-
Reactions were more common in younger people, women, those with a prior COVID-19 infection, and those who had a reaction after the first shot
-
Reactions were not affected by being on an MS drug, or type of MS, or how long someone has had MS
What do these study findings mean?
People with MS should not be concerned about having more serious reactions to the COVID-19 vaccinations compared to the general population.
What will we do next with this information?
The results of the study have been published in a variety of formats (see below) and have been shared with the National MS Society to inform their COVID-19 vaccination guidance for people with MS.
Learn more
Video: "Chat with Chat" interview with Dr. Farren Briggs
Infographic: COVER-MS Findings - COVID-19 Vaccine Reactions
Journal article (open-access): COVID-19 Vaccination Reactogenicity in Persons With Multiple Sclerosis
Journal article (open-access): Self-reported longitudinal COVID-19 vaccination reactogenicity profiles in persons with multiple sclerosis
Data: COVER-MS Data Dashboard
Poster: Details of the immune response substudy shared at the 2025 CMSC annual meeting