What were the goals of this study?
A “wearing-off effect” has been reported before by people taking certain disease-modifying therapies (DMTs) for MS. “Wearing-off” refers to a worsening of MS symptoms toward the end of the period between doses. This worsening is only temporary, and the symptoms generally improve again after the next dose is taken.
It’s possible that the length of time between doses might affect the chance of someone experiencing a wearing-off effect. Research into this effect could help determine if this is the case.
This study was designed to measure the changes in MS symptoms between doses for people treated with ocrelizumab (Ocrevus) and ofatumumab (Kesimpta). Both drugs work similarly in the body, by binding to a protein on B-cells called CD20 and eliminating these cells. However, Kesimpta is given once a month while Ocrevus is administered every 6 months.
Who led the study and what was the funding source?
This study was conducted in collaboration with Novartis, a pharmaceutical company that makes Kesimpta, and STATLOG Inc., a consultancy company that designs studies and conducts statistical analyses. Novartis provided the funding for the study.
How was the study conducted?
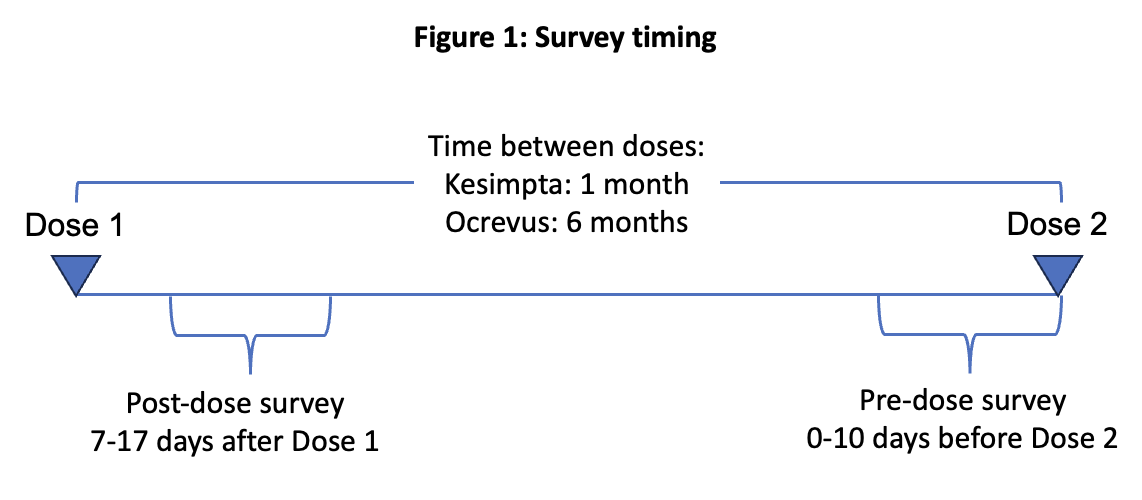
The study team developed a survey asking about the impact and severity of common MS symptoms such as fatigue, difficulty walking, pain, and depression. The survey also asked about ability to work and perform everyday activities, and whether the participant had noticed any wearing-off effects. Participation in the study consisted of completing a baseline questionnaire at enrollment, and then completing the study survey before and after the next two doses of Ocrevus or Kesimpta.
iConquerMS members who were taking Ocrevus or Kesimpta were invited to participate. In all, 60 Ocrevus users and 75 Kesimpta users enrolled in and completed the study. Participants needed to have been taking Ocrevus for at least one year or Kesimpta for at least 6 months in order to join the study.
What did we learn from this study?
The analysis focused on whether participants experienced any worsening of symptoms between their next two doses of Ocrevus or Kesimpta, based on surveys taken 7-17 days after the initial dose and up to 10 days before the following dose.
Around one-quarter of the Kesimpta participants (27%) compared with one-half (50%) of Ocrevus participants responded “Yes” to the question “Are you experiencing an increase in MS symptoms currently?"
In addition, participants taking Ocrevus were significantly more likely to report worsening of fatigue, mobility (walking) and cognition (thinking and memory) between the two doses than participants taking Kesimpta:
- Fatigue: 42% of Ocrevus users experienced worsening vs. 23% of Kesimpta users
- Mobility: 23% of Ocrevus users experienced worsening vs. 4% of Kesimpta users
- Cognition: 30% of Ocrevus users experienced worsening vs. 9% of Kesimpta users
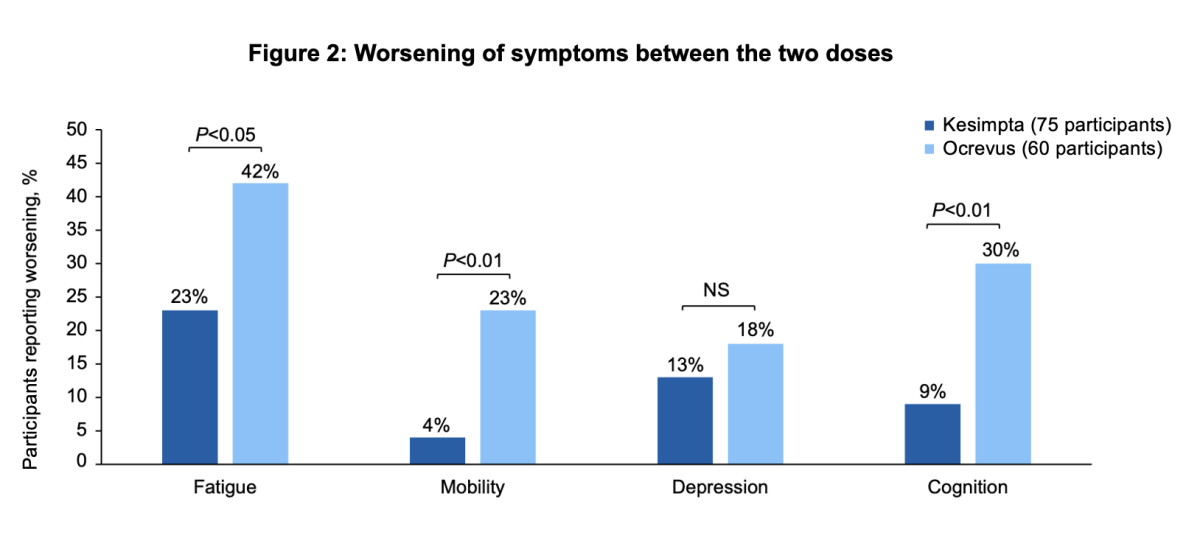
There was also a difference in the percentage of people reporting increased depression between the two doses (18% of Ocrevus users vs. 13% of Kesimpta users), but this was not significant.
What do these study findings mean?
These findings indicate that a shorter time between doses may reduce the possibility of experiencing a wearing-off effect with an anti-CD20 MS DMT.
What will we do next with this information?
The results have been presented at the annual meeting of the Consortium for Multiple Sclerosis Centers in 2025.
Learn more
Poster: Results shared at the 2025 CMSC annual meeting